TL;DR
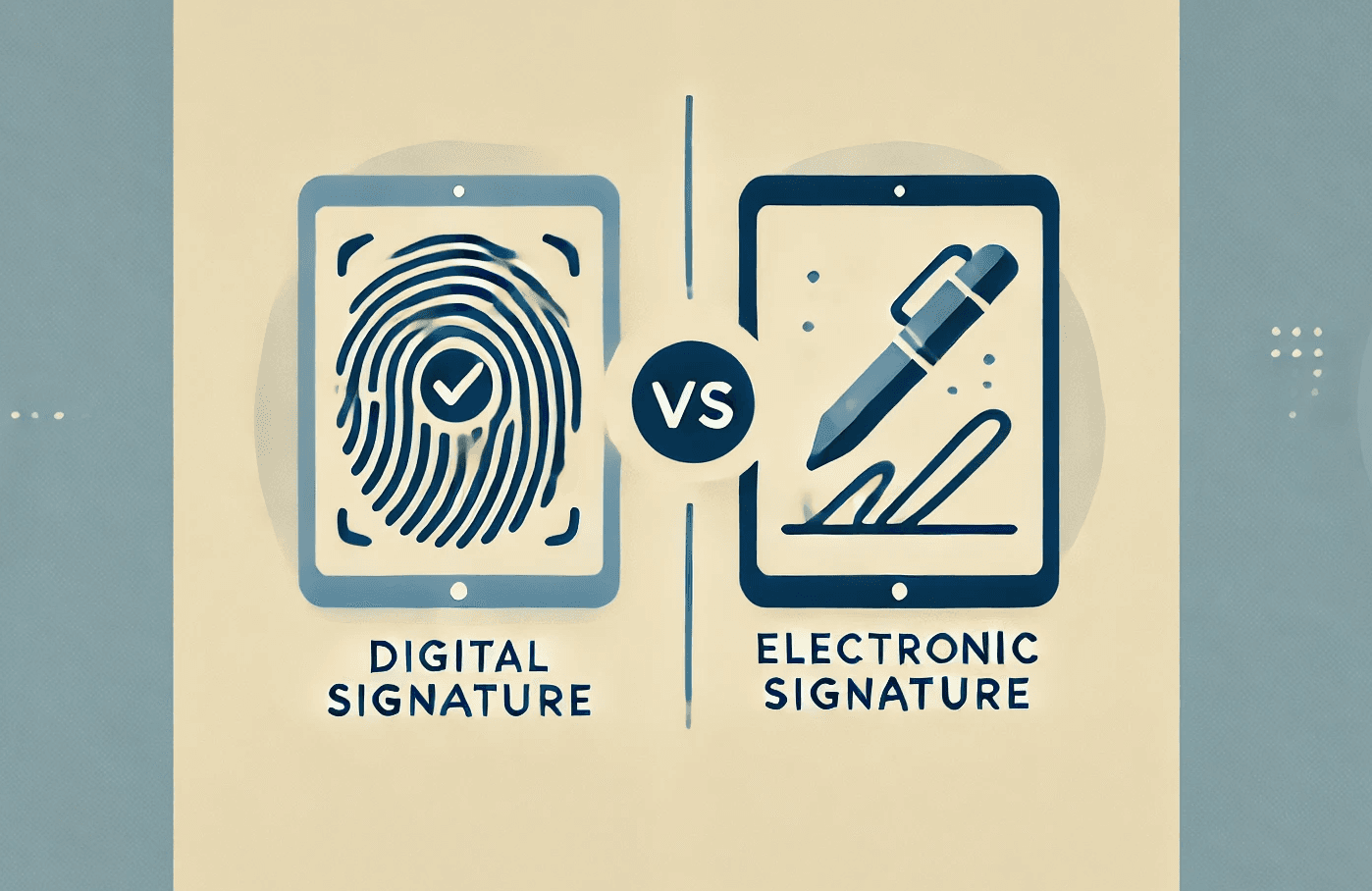
Electronic and digital signatures are essential for regulatory compliance in FDA-regulated industries. Electronic signatures verify the signer’s intent and identity, while digital signatures add cryptographic security to ensure document integrity. Both are key under 21 CFR Part 11 for ensuring that electronic records are trustworthy and legally binding.
What is an Electronic Signature?
According to the FDA’s 21 CFR Part 11 guidance, an electronic signature is defined as:
“A computer data compilation of any symbol or series of symbols executed, adopted, or authorized by an individual to be the legally binding equivalent of the individual's handwritten signature.”
(Subpart A—General Provisions, 11.3 Definitions, (7))
In many systems, electronic signatures are performed using the same username and password that a user employs to log in. This process of electronic signature generally follows these steps:
Steps for Electronic Signatures
-
Account Creation:
-
Each user is assigned a unique username and creates a password. This password is securely stored in the system using hashing, a one-way process that converts it to a fixed-length string, ensuring that the password itself is never stored in human-readable form.
-
Example of a hashed password:
a8b6c92f8f7c5c2b84885e4ecb1c31ebf3c9e5c5089eb3dfcd0a42b524c50d0
-
-
Document Signing:
- When signing a document, the user enters their username and password. The system hashes this password and compares it to the stored hash.
-
Verification and Authentication:
- If the hashed password matches the stored hash, the system authenticates the user and accepts the electronic signature.
This process confirms the user’s identity and intent, establishing the legal equivalency of an electronic signature to a handwritten one.
What is a Digital Signature?
The FDA also defines digital signatures under 21 CFR Part 11, which add a cryptographic layer to ensure the authenticity and integrity of the signed data. This type of signature is defined as:
“An electronic signature based upon cryptographic methods of originator authentication, computed by using a set of rules and a set of parameters such that the identity of the signer and the integrity of the data can be verified.”
(Subpart A—General Provisions, 11.3 Definitions, (5))
Digital signatures employ a pair of cryptographic keys—a private key for encryption and a public key for decryption—to provide a higher level of security. Here’s how a digital signature works:
Steps for Digital Signatures
-
Data Input and Hashing:
-
The signer prepares the document and generates a hash, which is a unique, fixed-length code representing the data. Hashing is one-way, so the original data cannot be recreated from the hash.
-
Example hash:
3f5c2e0e03d201f28d7b3451e8a3c2f4b4c4e1d7d0f8a1b2c3e4f5a6b7c8
-
-
Encryption with Private Key:
- The signer encrypts this hash with their private key, creating the digital signature. This private key remains confidential, ensuring only the intended signer can create the digital signature.
-
Attachment of Signature:
- The encrypted hash (the digital signature) is attached to the document, along with the metadata detailing the hashing algorithm and the signer’s public key. This allows future verifications without needing the original signing environment.
-
Verification Process:
- When the document is opened, the system rehashes the content and decrypts the original hash using the signer’s public key. If the hashes match, the document is verified as authentic and unaltered.
Digital signatures thus provide a higher level of verification, confirming both the signer's identity and that the document hasn’t been modified since signing.
Key Differences Between Electronic and Digital Signatures
Purpose
- Electronic Signature: Signer’s intent and identity verification.
- Digital Signature: Authenticates signer’s identity and ensures data integrity.
Security Level
- Electronic Signature: Basic security—typically username and password.
- Digital Signature: High-level security—public and private key encryption.
Verification Process
- Electronic Signature: Authentication by username-password match.
- Digital Signature: Cryptographic verification through key pairs.
Use Case Examples
- Electronic Signature: Suitable for routine approvals, internal documents.
- Digital Signature: Ideal for high-stakes documents and legal submissions requiring a higher level of security.
Why This Matters for 21 CFR Part 11 Compliance
In FDA-regulated environments, ensuring data integrity and traceability is essential. While electronic signatures are adequate for general tasks, digital signatures are often necessary for documents that require the highest level of security, such as those submitted for regulatory approval. Understanding and applying these differences helps ensure robust compliance with 21 CFR Part 11 and maintains the reliability of electronic records.
Conclusion
Both electronic and digital signatures play crucial roles in ensuring that electronic records are verifiable and secure. Organizations can leverage each type for its intended purpose, ensuring that routine and critical documents alike meet the necessary compliance standards. Proper implementation of these signature types strengthens trustworthiness in digital documentation, supporting an effective compliance framework under 21 CFR Part 11.

Practical Guide to 21 CFR Part 11
Your Essential Handbook for Navigating 21 CFR Part 11
“An invaluable resource for anyone working with computerised systems in pharma.”
Conor
Quality Assurance
Available on Amazon