
How to Check Network Configuration during IOQ Protocol
A step-by-step guide on how to check your network configuration for the IOQ Protocol using Command Prompt commands.
Learn everything you need to know about 21 CFR Part 11 compliance, including electronic signatures, electronic records, and implementation requirements for FDA-regulated industries.
Read More
Your Essential Handbook for Navigating 21 CFR Part 11
“An invaluable resource for anyone working with computerised systems in pharma.”
Conor
Quality Assurance
Available on Amazon

A step-by-step guide on how to check your network configuration for the IOQ Protocol using Command Prompt commands.
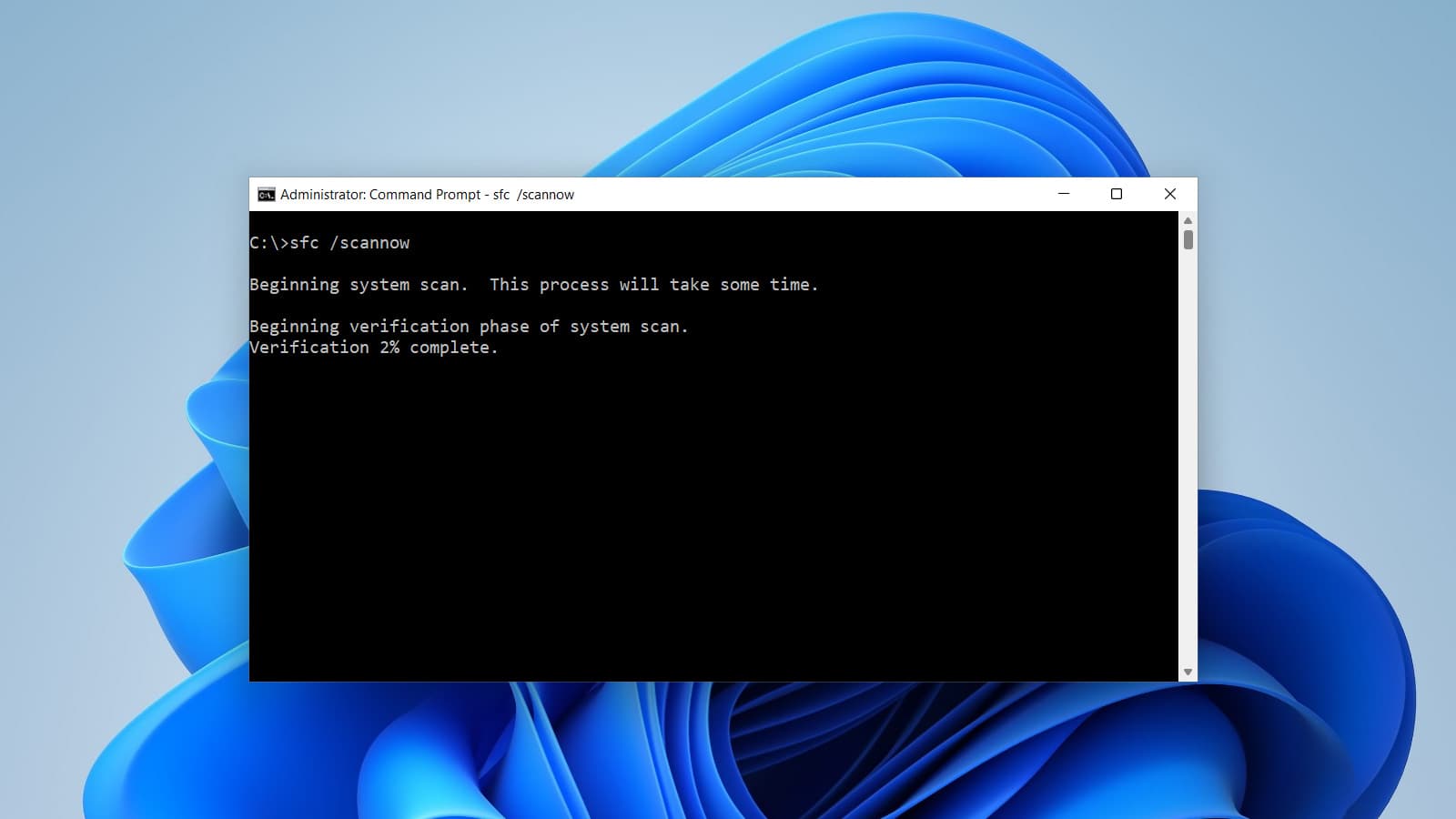
A comprehensive guide to essential CMD commands useful during IOQ execution in the context of computerized system validation in the pharmaceutical manufacturing industry.
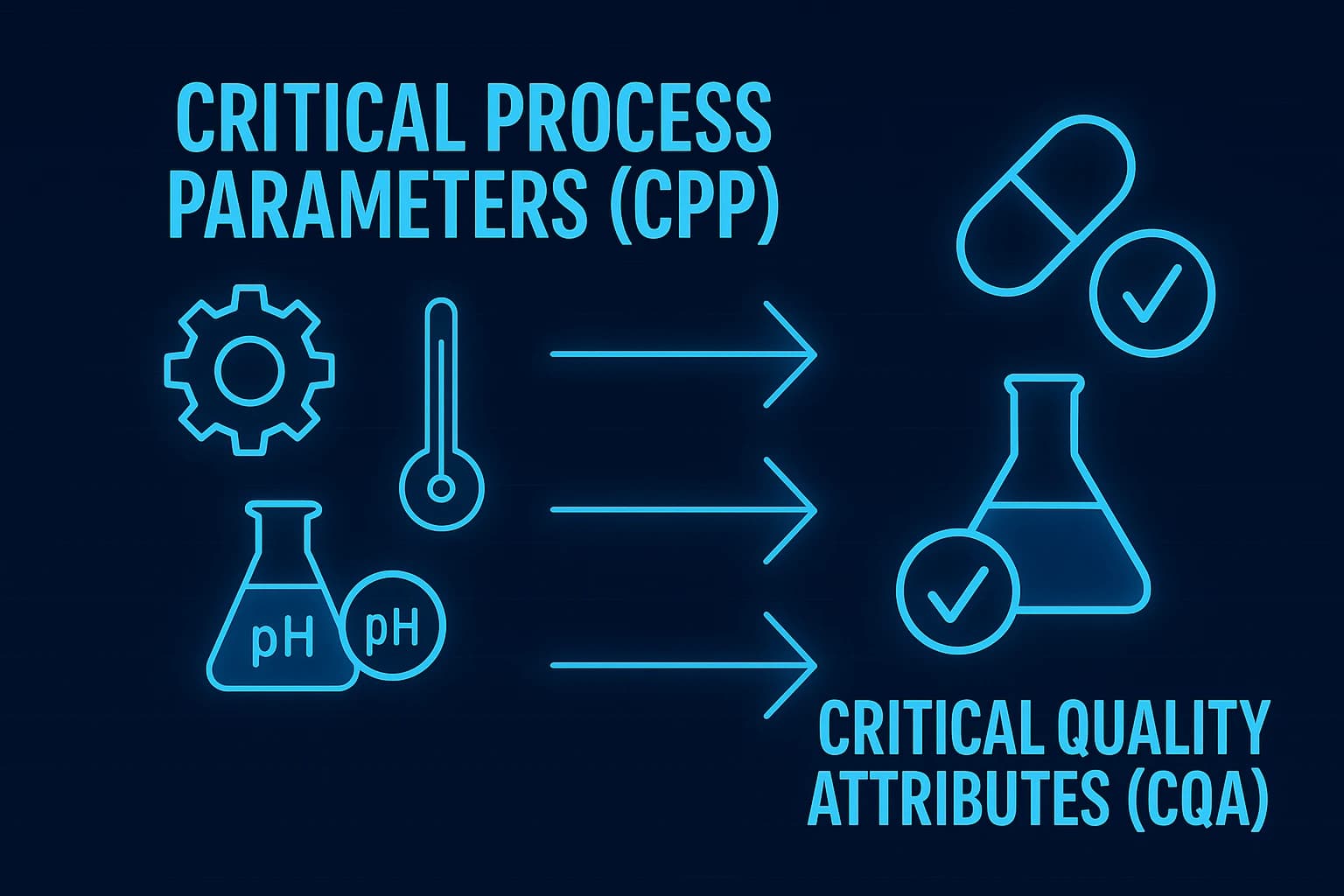
Explore how Critical Process Parameters (CPPs) influence Critical Quality Attributes (CQAs) in pharmaceutical manufacturing to ensure product quality and compliance.

Understanding the critical differences between electronic signatures and event attribution in audit trails under FDA's 21 CFR Part 11 for compliance and security in the pharmaceutical industry.

Learn how to comply with EudraLex Annex 11, Section 7.2 by ensuring your backups can be restored effectively, focusing on validation and periodic monitoring.

Discover the role of time-stamped audit trails in 21 CFR Part 11, essential for ensuring compliance and data integrity in electronic records.

Explore the relationship between 21 CFR Part 11 and FDA predicate rules, focusing on compliance requirements for electronic records in regulated industries.
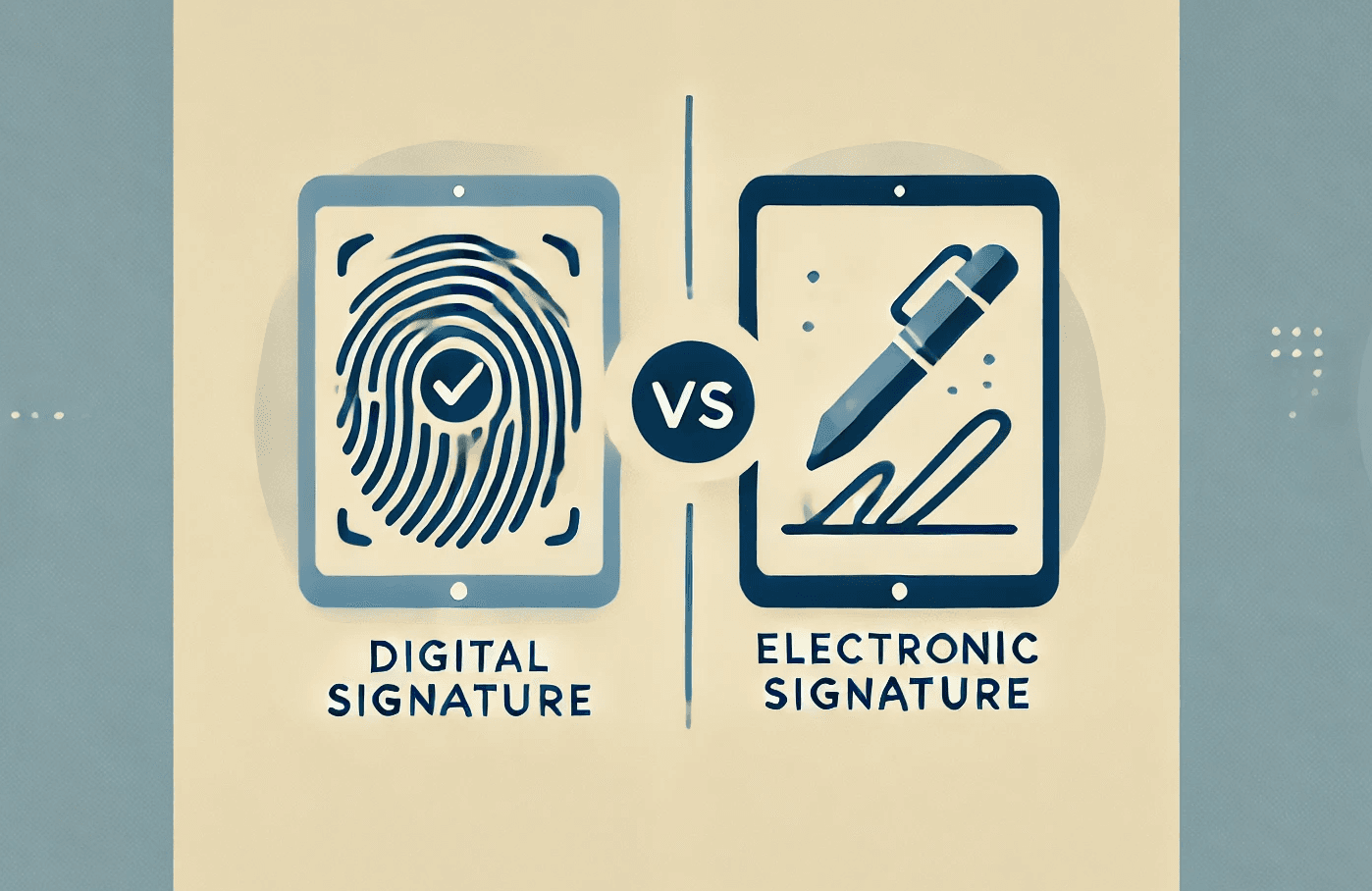
Learn the differences between electronic and digital signatures under 21 CFR Part 11 and their importance in regulatory compliance for FDA-regulated industries.